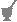|
Mixing Solids: Random vs Ordered Mixing, Part II
Loyd V. Allen, Jr., PhD, RPh
Editor-in-Chief
of
International Journal of Pharmaceutical Compounding
and
Remington: The Science and Art of Pharmacy, Twenty-second Edition
As we discussed in the previous newsletter, mixing plays a vital role in obtaining uniform dosage forms, and we listed both "random mixing" and "ordered mixing." This issue will discuss ordered mixing.
The concept of ordered mixing involves the mixing of small cohesive particles to a considerable degree of homogeneity. A basic principle is that fine particles will adhere, especially to larger particles; the adhesional forces involved may be electrostatic or surface tensional. In a random mix, each individual particle can move independently and randomly. In an ordered mix, the ordered "unit" moves as a whole, not the individual particles.
An ordered mix is obtained when the drug, in a finely dispersed state, adheres to the surface of substantially larger carrier particles. When the carrier particles are water soluble (mannitol, lactose), and come into contact with water which dissolves the carrier, the active drug particles become surrounded with water and are released, or dispersed, as microscopic drug particles.
The surface area ratio (Rs) is a measurement of the quantity of particles of active drug which will adhere to the surfaces of the larger carrier particles; i.e., the ratio of the projected surface of the adherent particles to the total external surface area of the carrier particles. The Rs value is defined as:
Rs = (API surface area covered on the carrier) / (Carrier total surface area)
An Rs=1 is when the surface of the carrier particle is entirely covered with active drug particles. When the amount of active drug used is very high and the surface area is covered by a lot of the drug (the Rs value is high), then the dissolution rate is decreased.
Generally, the dissolution rate decreases markedly at an Rs value as low as about 0.5 and that at values approaching or exceeding Rs=1 the dissolution rate has decreased to such an extent that none of the advantages are realized. This is presumably because the surface particles prevent rapid penetration of the water to the underlying carrier substance and subsequent rapid dissolution.
The process of mixing cohesive, interacting particulate systems follows a "disorder to order" concept and probably occurs widely in actual systems. The requirements for ordered mixing are different from those required for random mixing. Ordered mixing requires larger carrier particles and smaller active-drug particles.
Ordered mixing is particularly useful in the manufacture of capsules and tablets containing potent materials because uniformity of mixture depends upon larger units, not smaller particles. The standard deviation of ordered mixtures tends to be very small.
If one of the constituents of a powder mix is added as a fine, often micronized form, then on mixing, the larger particles (carrier particles) may adsorb some of these very small particles on to active sites on their surface and these are held tenaciously. It is now recognized that ordered mixing is an important factor preventing the segregation of mixes of a drug with one or other of the direct compression bases.
Ordered mixing probably comes the closest to yielding the perfect mix and may be obtained by (1) mechanical means, (2) adhesion, and (3) coating. The major difference between the mechanical and adhesional and coated ordered mixing is the degree of force holding the ingredients in each type of the ordered units together.
Examples of "ordered" mixtures are dry blends of sucrose and antibiotics that can be reconstituted with water to provide antibiotic syrup formulations. Sorbitol can replace sucrose to prepare sucrose-free formulations for diabetic patients. During blending, a fine powder of an antibiotic is adsorbed onto the surface of coarse particles of sorbitol.
Another area where ordered mixtures are used is in powders for oral inhalation. These powders are most commonly binary ordered mixtures composed of micronized drugs and coarse carriers (often coarse lactose). One example is albuterol sulfate powder for oral inhalation. This product is a powdered mixture of microfine drug (95% of particles having a diameter of 10 µm or less) and lactose and is contained in a hard gelatin capsule.
Preparation of ordered mixtures involves regular manufacturing/compounding equipment and methods. Particle-size reduction to the desired size range for each of the ingredients is followed by blending processes for pre-determined time periods to obtain an "ordered mixture." On a small scale, this may require up to 25-50 hours of mixing; on a larger scale, the time is decreased because of the mechanics involved and higher shear rates.
An illustration of an ordered mix would be as follows when looking down on an individual, single carrier (0) particle with adhered smaller (x) particles cut in half (Example 1); next are complete particles looking at the carrier surface of an ordered mix with an Rs value of 0.25, 0.5, 0.75, and 1.0 (Examples 2 through 5).
| Example 1 | Example 2 | Example 3 | Example 4 | Example 5 |
|---|
| xxxxxxxx | x000000x | x0x0x0x0 | xxxx0xx0 | xxxxxxxx |
| x000000x | 0x0000x0 | 00xx00x0 | 0xxxxx0x | xxxxxxxx |
| x000000x | x000x000 | x0x00x0 | xxx00xxx | xxxxxxxx |
| x000000x | 000x0x00 | x00x0xx0 | xx0xxx0x | xxxxxxxx |
| x000000x | 0x00x000 | 0x00xx0x | 0xxx0xxx | xxxxxxxx |
| x000000x | 00x0000x | 00xx00xx | 0xx0xxxx | xxxxxxxx |
| x000000x | 0x00x000 | x00xx00x | xx0xxxx0 | xxxxxxxx |
| xxxxxxxx | 0x0000x0 | 0x0x0x0x | 00xxxxxx | xxxxxxxx |
As is evident from these examples, example 1 shows the active-drug particles adhered to the outer surface of the halved particle and the single-carrier particle in the center; example 2 contains a carrier particle that is 25% covered with active drug (x); example 3 is 50% covered; example 4 is 75% covered; and example 5 is totally covered. Any additional drug would simply stack up on the already adhered particles or would remain free.
In summary, especially for potent drugs, the use of an ordered mixing process is advantageous for homogeneity.
|