|
Continuing our BCS topic, this week we will look at an extension of the BCS and the Biopharmaceutics Drug Disposition Classification System (BDDCS). The BDDCS was developed in 2005 incorporating the effects of food, absorptive transporters, efflux transporters, and routes of elimination on overall drug absorption and bioavailability for immediate-release oral dosage forms as it considers more than just permeability. The BDDCS classification system is described as:
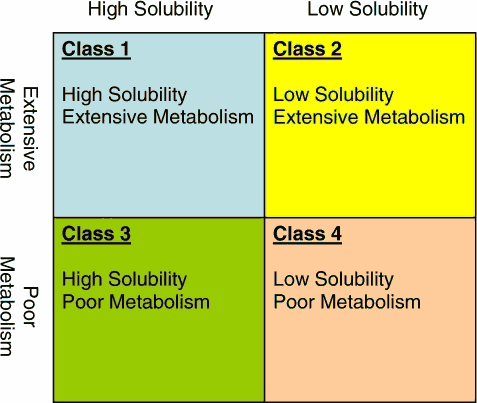
| Class 1 | High Solubility | Extensive Metabolism |
| Class 2 | Low Solubility | Extensive Metabolism |
| Class 3 | High Solubility | Poor Metabolism |
| Class 4 | Low Solubility | Poor Metabolism |
A diagram of the BDDCS is as follows:
If additional information is added related to pharmacogenomics, the diagram becomes:
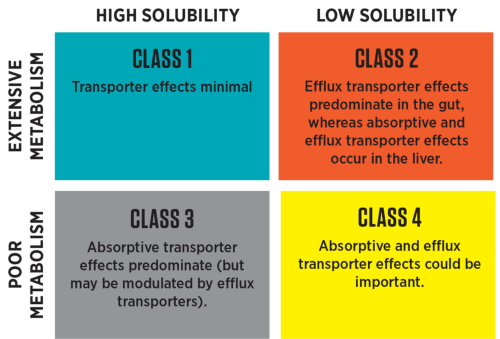
The BCS and BDDCS are complimentary and do not compete; they are classification systems that improve, simplify, and speed drug development. Although similar in classifying drugs into four categories using the same solubility criteria, they differ in the criteria for permeability and are used for different purposes; there is a very strong correlation between intestinal permeability rate and the extent of metabolism. In general, the classification of drugs between BCS and BDDCS differs only about 5% to 10%.
More on the BDDCS system next week.
Loyd V. Allen, Jr., PhD, RPh
Editor-in-Chief
International Journal of Pharmaceutical Compounding
Remington: The Science and Practice of Pharmacy Twenty-second edition
|